Almost everyone knows about the existence of precious metals. These include silver, gold and platinum. Moreover, not everyone knows about the existence of a platinum group. Its representatives are also considered noble.


Features
Platinum metals - This is a group of 6 elements that are located next to each other in the periodic chemical table. Each of their elements in the group is considered to be noble. This is due to the following chemical and physical properties.
- Experts note a low concentration of platinum metals. The number of deposits is small. This characteristic also applies to chemical elements that are traditionally considered rare and expensive.
- Representatives of the above group possess the properties of the following metals: rhodium, osmium, palladium, ruthenium.
- In the process of studying platinoids, there was an excellent resemblance in the atomic structure to the elements indicated above.


Scientists working in the field of chemistry have divided all platinum metals into two groups, called triads.
Separation is by weight.
- Group No. 1. These are the easiest representatives. These include palladium, ruthenium and rhodium.
- Group number 2. The remaining 6 metals are iridium, osmium and platinum itself. These are already heavy metals.


Metals and their properties
The metals of the above group have their own designations. The list is like that.
- Ruthenium - Ru.
- Rhodium - Rh.
- Palladium - Pd.
- Osmium - Os.
- Iridium - Ir.
- Platinum - Pt.


Note: all designations are arranged in a certain order, according to atomic weight.
From smallest to largest. All platinum metals have similar characteristics.
- The first similarity is in appearance.Almost all elements except osmium have a light shade (a combination of white and silver colors). Osmium has a light bluish tone.
- Metals have high resistance to many reagents. In this case, platinoids are effective catalysts.
- With their help, they control various chemical processes, control the rate of oxidation, and also monitor other reactions. This behavior of metals is considered surprising and paradoxical.

The properties
No oxidation forms on the surface. Thus, inertness is clearly demonstrated. According to experts, it is especially noticeable in platinum. When studying the representatives of the platinum group, it was impossible to ignore the melting point. The lowest value for palladium and is 1554 degrees. Osmium has the highest value. Its temperature is 3 thousand 27 degrees Celsius.
The next same property is infusibility. This characteristic indicates excellent wear resistance. Despite the resemblance, the physical qualities are different. Depending on these indicators, special techniques are used during processing. Ruthenium and osmium are very fragile metals and require special care.
High ductility is an indicator of palladium and platinum.


Where are they mined?
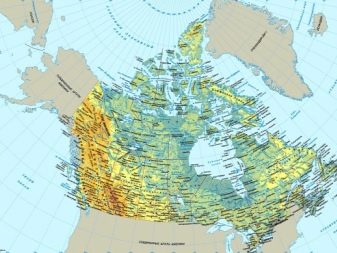
Deposits of platinum metals are usually scattered throughout Canada and South Africa. In these regions, the extraction of minerals occurs in a standard mine way. As practice shows, most platinoids are mined by extracting elements from nickel sulfide minerals or copper ores. In the work using flotation separation. In the process of metal processing, the obtained concentrate is melted, as a result of which a special mixture is obtained. The volume of platinum metals is from 15 to 20% of the dry residue.
In some cases, the extraction and processing process may vary. Sometimes gravity separation is used in factories. In this case, the amount of necessary chemical elements increases to 50%. This processing option eliminates smelting. Although rich PGM deposits are rare, some deposits are also located in Canada, China, Australia and Finland.
There are other sources, however, the share of their production is only 0.3% of the total mass mined on the planet.

Where are used?
Platinoids have found their application in various fields. The universal properties of metals of this group are actively used today. Pure Platinum itself is very soft and supple. In this condition, it is very susceptible to various damage and defects. To increase the hardness and wear resistance of the precious metal, various elements are used. Platinum is fused with other chemical elements.
Jewelry made of platinum is highly valued and worth more than gold. They gained particular popularity in Japan. Residents of the Land of the Rising Sun call such products "hakkin." The main jewelry alloy is platinum and accounts for 90% of the total mass. The remaining 10% is palladium. It is easy to work with, including soldering and other processing.


Also to increase hardness, precious white metal is combined with ruthenium. The addition of this element increases the resistance to the oxidation process. IPY found its application in the manufacture of forged products. In this case, an alloy consisting of copper, platinum and palladium is used. This option is more affordable in comparison with the composition of two elements: platinum and palladium.
Special alloys that are made using platinoids are widely used in the manufacture of thermocouples. This is a special device with a wide range of applications. Its main purpose is to change high temperatures (the maximum value is up to 1800 degrees Celsius above zero).Some representatives are applied in pure form. As a rule, they act as additives to other metals from the platinum group. Palladium has found its application in the production of electrical equipment, as well as in modern dental alloys.

Catalysts
Over 40% of all platinum produced, which is produced abroad, is used as a catalyst. This amazing and useful property was identified in the first part of the article. Almost all metal (approximately 90%) found its application in the production of exhaust systems for automobiles. The precious material, as well as rhodium and palladium, acts as a protective coating for honeycomb structures and other elements. The metal layer protects against oxidation processes, keeping the equipment safe and sound. Entering a chemical reaction, aggressive components are converted into safe compounds and substances.
Chemical elements can perform the task of an effective catalyst not only as a coating, but also in the form of a hot metal mesh. In this case, a reaction occurs between air and a toxic substance - ammonia. The result is nitric acid and nitric oxide. To obtain other substances add various components.
Another area that can not do without the use of PGM is oil production. This is a global industry that points to the value of platinoids and their importance in industry.


Other uses for metals are.
- In Russia, palladium is made investment coins. They began to do this after a coin from pure palladium was issued in Soviet times. Denomination - 25 rubles.
- High-voltage equipment is also not complete without the use of PGM. In the manufacture of this type of electronics, wear-resistant and reliable contacts are needed that will be resistant to negative external influences. Despite the development of new technologies, there is currently no valid alternative to platinoids.
- In the process of manufacturing tools and devices for working in difficult conditions such metals are also actively used. To work in an aggressive environment, the equipment must be strong, wear-resistant and durable. It is these characteristics that it receives from platinum metals.
- To significantly increase the resistance of titanium to corrosion, a little palladium is added to it. Also, this element of the platinum group is often mixed with steel.
- In medecine active compounds are also actively used. This practice has been applied before and remains relevant today.
- Do not forget about platinum foil. This material is used to protect the device of chemical reactors.
- Alloy of silver and palladium is actively used. in low-current electronics.


About how this precious metal is still used, see the next video.










