Due to many unique properties, silver is used not only in the manufacture of jewelry and expensive cutlery. Great demand has a procedure called silvering of parts, during which objects are coated with a thin layer of light metal. Let us dwell on the galvanic method, which can be implemented at home.

Process features
Silvering of parts in a galvanic way (electroplating) is very popular among professionals who have long been working with metals, and ordinary people who want to coat another object with a layer of precious metal. This technique has found its application in the coating of copper figurines. Using electroforming, you can create objects of any complexity. The technique is also widely used to repair damaged surfaces and elements.
The main feature is immersion in a special substance (electrolyte) of the object necessary for processing. For work, a liquid is used, in which there are metal salts. Without them, coverage will not work. During the procedure, salts begin to dissolve in water into positive and negative ions. This process is called electrolysis.
It is worth noting that electrolytes are wonderful conductors. During immersion of the object in the liquid and connection to the power supply, electric current will constantly flow through these particles. Electrolytic action triggers the necessary processes.

Necessary tools and materials
Before proceeding to silvering, it is necessary to prepare the following.
- Reliable capacity.
- DC power supply.
- Scales (you can use pharmacy or jewelry, so that you can work with compounds and small items).
- Electrolyte.
- Electric stove.
- The wire.
- Set of electrodes (anode and cathode).
You may also need silver nitrate (a lapis pencil, which can be bought at any pharmacy) and sodium salt (an element with sodium cyanide).
The exact list of the necessary tools and materials depends on how the silvering will be performed, and what objects need to be coated with a thin layer of metal.
When choosing a power source, select a device with the ability to adjust the electric year. If you intend to work with objects the size of a large apple, you need to use a rectifier that is designed for an output current of up to 5 A. In the event that you need to process a small object, 0.5 A or even less will be enough.
When using the galvanic technique, 12V chargers can also be used. Such devices are available in almost every home where there are gadgets. Old-type batteries can also be used as the power source needed for the silvering process. If it is necessary to reduce the current flow, experienced specialists recommend using shunting.

The bath that you will use in your work should be strong and roomy. Two other important characteristics are resistance to reagents, electricity and high temperatures. A glass container with a wall thickness of 0.5 centimeters is great. In the house you can find an aquarium or a wide-necked jar.
In order for the result to be positive, it is necessary to accurately weigh the components used. In this case, accurate scales, preferably electronic, are useful. It’s convenient to work with them. It is recommended to check the device before use.
To perform the procedure, precious metal plates will be needed. They act as electrodes. These are donors, molecules from which will pass to the processed object. Through them, the current passes to the electrolyte. It is better to choose stranded wires made of copper, cross-section is 2.5 square meters. mm If you need to perform heating during operation, you can use a conventional microwave oven.
An oven (electric or gas) or an electric stove is also suitable.

Safety precautions
When performing silvering, it is imperative to adhere to safety regulations. Otherwise, you can not only ruin the equipment and supplies, but also cause harm to your health. In the room where the work is performed, ventilation must be provided. Open the windows for the flow of fresh air and its circulation. If the room does not have windows, turn on the air conditioner or install a fan.
Be sure to protect your hands and respiratory system. Use rubber gloves and a respirator. Use quality products. Also put on your old clothes, which is not a pity to spoil. It is forbidden to eat food or water during operation. Particles of dangerous and aggressive reagents may remain on hand.
We mention the possible dangers in the process.
- Evaporation of chemical compounds.
- High temperatures.
- Electricity.
- Destruction of used containers.
- Contact with caustic compounds on exposed skin.

Working process
Preparation
Before starting silvering, it is necessary to properly prepare the product for processing. Items must be thoroughly cleaned of dust, rust, greasy traces and other things. You can choose a manual method of cleaning or use special equipment, such as a grinder. When using abrasive products or hard metal sponges, be careful not to damage the product.
If the workpiece has deep scratches, they must also be thoroughly cleaned. Otherwise, the metal atoms do not form a sufficiently strong bond and simply become a precipitate. After the first stage of cleaning has come to an end, the product must be placed in a special solution.To do this, you need an acid or alkaline composition. At this stage, you need to act carefully so as not to get a skin burn.
When silvering steel products, they can be immersed in sodium phosphate for several minutes, heating the composition to 90 degrees Celsius.
To clean copper items or products made of its alloy, you can use conventional detergent compositions or solutions based on soda. Another option is the use of sodium phosphate, but in this case it can not be heated. In some cases, cleaning using chemicals may not work. Leftovers may be like that.
- Traces of paint or enamel.
- Burnt frozen fat that has eaten into the surface.
- Deep corrosion damage.
- Slag.
- Residue Resin.
If after the first cleaning it is not possible to achieve the necessary results, you need to perform this procedure several more times. You can not start silvering an object until it is completely cleaned.

Main stage
To perform the process at home, you can use a special saline solution. Muric acid is the main component for work (hydrochloric acid HCl). It is also called soldering. The composition has similar properties with sulfuric acid. The silvering method is as follows.
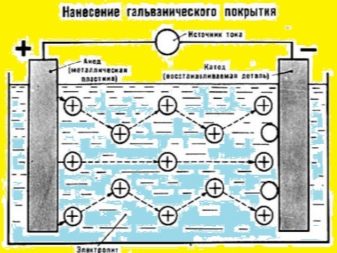
- First, a steel plate and a silver blank must be connected to the supply clamps. Be sure to observe the polarity in the process. Silver needs to be connected to the plus, and the second element to the minus.
- The working tank is filled with clean water and hydrochloric acid. The required proportions are 5: 1.
- Two elements (the donor and the object to be coated with silver) are placed in the composition. The clamp on the parts is connected to a place that does not need silvering. It is also possible to move the clamp throughout galvanization.
- To make the silver layer smooth and homogeneous, the composition must sometimes be mixed.
- Note: during the preparation of the electrolyte, the acid must be added to the water gently, with a fine stream. After the liquid is gently mixed with a glass stick.
- It is necessary to arrange a small distance between the positive and negative electrodes. In the process, the object is covered with a thin layer of precious metal, it is difficult to arrange a thick coating. This process may take several hours.

When carrying out the procedure at home, it is necessary to determine in advance which chemical reaction should result. During the selection, you need to consider the metal with which you want to perform silvering. Lead is great as an anode when coated with precious metals. Do not forget to update the composition in which the galvanization process (electrolyte) is performed.
A visual example of applying a thin and even layer of silver to the surface of another material can be found in any home. This is an ordinary mirror. By combining glass and precious metal, it is possible to achieve the desired result.
Also, the silvering technique is great for brass, copper and various items from their alloys.
When working at home, the selected workpiece must be coated with nickel and only then proceed with silvering. To prepare the necessary solution, you need to prepare the following components:
- 40 grams of iron-cyanide potassium and the same amount of potassium carbonate (soda ash);
- 70 milliliters of ammonium hydroxide in the form of a liquid solution;
- 10 grams of silver chloride;
- 15 grams of edible salt (sodium chloride);
- a liter of distilled water, while boiled or tap water is not suitable due to the presence of external impurities;
- you will need a rod of a graphite construction pencil, it will play the role of the anode.
The attached circuit will help to do the work. It clearly demonstrates the process. Here is another visual diagram that details the essence of the method described in the article. Everything is very clear on the image.

Completion
After the process has come to an end, it is necessary to get rid of the remnants of the chemical solution. They need to be poured into the sink and washed with plenty of running water. All parts used during operation must be wiped dry. Use thick paper towels or a soft cloth.
Take off your work clothes. Throw away disposable protective equipment immediately; they must not be reused. Wash hands thoroughly with soap and water. Leave the room to ventilate until the air is completely clean. It is also necessary to thoroughly wash the clothes, it is advisable to rinse them thoroughly in order to completely get rid of the particles of reagents. Remember to clean containers and tools that were used in the process and come in contact with hazardous and aggressive components.











