Substances that exist in nature - a practically inexhaustible topic for stories. Moreover, not all of them are equally well known. It is necessary to find out, at least for general development, the main moments associated with platinum, its properties and application features.

What it is?
Those who graduated from school a few decades ago would surely say that platinum is the metal of a side subgroup of the 8th group of the Periodic Table of the Elements. However, this classification is outdated, and now platinum is assigned to the 10th group of elements.
Its atomic number is 78. It is a mineral extracted in large mines. And also sometimes there are platinum nuggets of various sizes.
It is impossible to say how this metal looks in nature. Indeed, in its pure form it can only be obtained artificially. Platinum ores have only small inclusions of the basic substance. From a physical point of view, they are isomorphic mixtures of Pt with:
- copper;
- iron;
- nickel;
- silver
- various platinum group metals.

Origin history
Platinum ores are everywhere in a diffuse state. Most of them are concentrated in the New World. because the honor of the discovery of platinum belongs to the ancient Indians. When exactly they began to mine this metal, even experts do not know. But after the discovery of platinum by Europeans, its role in the economy was more likely negative.
The name of the metal itself comes from the Spanish "silver". It was actually used to counterfeit a full-fledged silver coin.
No wonder in 1735 in Spain they decided to ban the import of previously mined platinum into the metropolis. What was mined again in Colombia was ordered to be carefully separated from silver and flooded in rivers. And everything that they had managed to bring to Spain itself was drowned in the sea in a festive atmosphere.

But it is curious that less than half a century has passed, as Madrid, on the contrary, has set a course for the enhanced import of platinum raw materials. Then they began to use it on a state scale to falsify coins from other precious metals. By 1820, according to various sources, in Europe it turned out from 3 to 7 tons of platinum. It was from it in France that the standards of meter and kilogram were made. But anyway, it still had no serious use.


Composition and properties
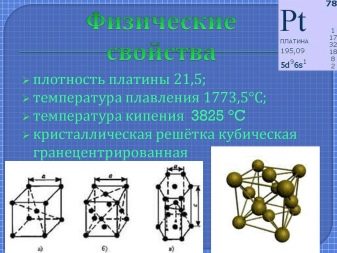
The specific gravity of pure platinum is 21.45 g per 1 cubic meter. see. It melts at a temperature of about 1768 degrees Celsius. Boiling (evaporation) occurs at 3825 degrees. It is these temperature characteristics that for a long time did not allow not to isolate pure metal, much less to establish its use. In addition, platinum is harder than gold and silver; it is rather difficult to process it mechanically.
This metal is forged and ductile. Its tensile strength is very impressive.
It is practically impossible to oxidize platinum or to affect it with any alkali.

It dissolves only in:
- royal vodka;
- liquid bromine;
- heated sulfuric acid (but extremely slowly).



Important: after heating, the reactivity of the metal increases significantly. But he does not magnetize.
Therefore, bringing it to a magnet to separate it from silver or gold does not make sense. You just need to understand that pure platinum jewelry does not exist. They may contain various concentrations of iron and nickel, and just these impurities are completely responsive to the magnet.
Curious that in the microworld, platinum may well have magnetic properties. Physicists in the experiment managed to find them in the atomic layer of a metal; The opening was announced in 2018. To give the substance ferromagnetic properties, it was necessary to use ionic liquid - a new kind of substance specially created during research. As for the color of platinum, in nature it is painted in a silver-white tone. Dark gray samples are sometimes found.

Where and how is it mined?
It is important to extract platinum ore from the bowels of the earth, there are several ways for this.
Place of Birth
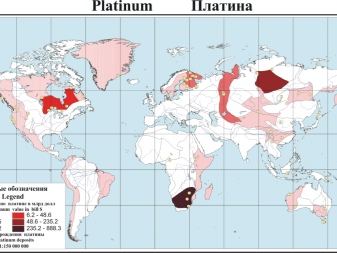
Despite the initial discovery of platinum deposits in South America, the largest field in the world in the 21st century is located in Africa. If exactly, then in South Africa. The Bushveld complex is a giant accumulation of platinum group metals; it is believed that it appeared 2 billion years ago as a result of volcanic processes. The shape of the South African field resembles a "plateau" with a diameter of 370 km. The complex is composed of several horizons that are directed inland.
Moreover, the thickness of deposits in 2 out of three horizons is only about 1 m. The Platrif horizon, which is being actively developed now, has a thickness of 5-90 m in different places. It is mined in an open way. The complex was opened in 1924. It is from here that the world ¾ of all platinum mined goes to the world market.

It is curious that in Russia (more specifically, in the Urals) in the first half of the 19th century this metal was mined more than anywhere else in the world.
But even today, our country is included in the list of leading countries for the extraction of platinum ores. True, it is already in second place, behind South Africa by 5.8 times. Precious metal is also sent from Russia for export. The third place in the world ranking is occupied by Zimbabwe, where they produce about 3 times less platinum (9 tons).
And also platinum is mined:
- USA (6000 kg);
- Canada (approximately 5000 kg);
- other states (6100 kg together).


Mining methods
This metal can be extracted both open and mine. Quarries are mainly located where secondary placers are found. In these places, platinum was deposited after mechanical destruction of the primary deposits. But geologists have long discovered that the bulk of it is concentrated in underground strata of nickel ores. Mine mining differs little from work on other metallic minerals; it is worth noting only a significant proportion of manual labor.
One way or another, the extracted ore has to be enriched. In the originally extracted form per 1000 tons, only 1-6 kg of target raw materials are accounted for.
After enrichment, its concentration rises by 3 orders of magnitude. At the Bushveld complex, the extraction of 1 kg of platinum ultimately (taking into account technological losses) requires raising 500-1500 kg of ore. Then the semi-finished product is processed in metallurgical treatment furnaces and special converters; but the final result is achieved only after refining, when the metal concentration is 99.5%.


Comparison with other metals
Platinum is better than gold because it is stronger than it. Therefore, the service life of platinum products is much higher. Scratching them is much harder. As a result, these types of jewelry (and not only) products are less likely to be repaired. Platinum "aged" differs from gold even more.
She, thanks to the patina, acquires a gray color with a matte sheen. Many owners try to get rid of this effect and polish their jewelry. According to other people patina makes a precious metal, if not even more valuable, then at least more interesting. Not seeing live, to make the right decision is unlikely to succeed. The rhodium layer on white gold will gradually wear off, and it will become much yellower.

Silver is softer than platinum, and the latter, of course, is much heavier. The durability of silver products is also not too great.
They also gradually fade, and they will have to be cleaned systematically. After all silver is chemically active, and it will inevitably react with the most common substances, even atmospheric oxygen. However, all this difference in favor of platinum is overshadowed by the fact that it is noticeably less accessible than gold and especially silver.


Tungsten is like platinum. But it is significantly cheaper, while the strength of tungsten products is also great. The problem is that tungsten is much harder to process, giving it a normal shape. Remaking a tungsten ring and adjusting its size is not an easy task even with 21st century technology. The same difficulties arise when using titanium imitations.


Alloys
There are quite a few types of platinum alloys. Jewelry for the most part is made of 950 metal, which contains 95% pure platinum. Sometimes you can find the 900th alloy. Its obvious disadvantages are not too saturated color and inexpressive brilliance. Therefore, in aesthetic terms, he loses a lot to the 950th metal.
Alloys of platinum with iridium are widespread. The more this second component, the more refractory the compound. The crystallization interval of platinum-iridium alloys is relatively narrow. The hardness and strength of the substance also increase significantly.

For various purposes, platinum can still be fused with:
- copper;
- ruthenium;
- palladium;
- nickel;
- rhodium.



Application
A very large proportion of platinum is used in industry. Technologists highly appreciate the property of this metal to accelerate various chemical reactions without diverging. Now it is sometimes used in medicine, primarily for dental prosthetics. Already in the middle of the last century, such applications accounted for several percent of all platinum mined. The amount is gradually growing.
But the undisputed leader in terms of volume has been and remains the jewelry industry. Every year she uses at least 50 tons of platinum.
Its use in the production of nitric acid (as an oxidizing agent of ammonia) is also widespread. However, in this case, platinum-rhodium alloy is more often used, and not pure metal. The reasons for this preference are of interest only to technologists and are beyond the scope of this article.Another precious metal is used in the production of sulfuric acid, hydrogenation of hydrocarbons, acetylene, ketones.

But Platinum is also widely used in the oil refining industry. It is an excellent catalyst accelerating the production of gasoline. In distillation columns, not a net is placed, as is sometimes thought, but finely divided platinum powder. It is more durable and molybdenum, and vanadium. And also in favor of platinum is evidence of increased efficiency.
Platinum-iridium alloy is in demand when creating high-quality contacts in electronic products.
Platinum can be used in resistance contacts of electric furnaces. You can meet her in many other electrical contacts.
Platinum-cobalt alloy is needed to create magnets that combine compactness and tremendous performance.

Every day, many motorists implicitly use platinum. In cars, it is mainly found in catalysts. This metal helps reduce exhaust emissions, thereby improving the urban atmosphere. The platinum coating in the catalyst is applied to a monolithic ceramic element.
The space industry and the aircraft industry need platinum electrodes for fuel systems.
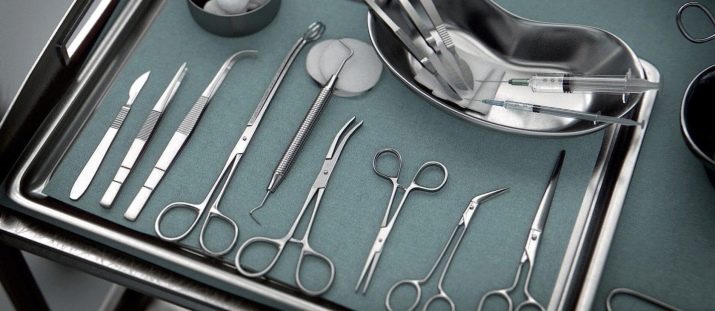
Returning to medicine, it is worth noting that platinum-based make unique surgical instruments. They can be disinfected with alcohol burners without spending special reagents. Dentists love to work with tools that spray a thin layer of platinum. Platinum-iridium electrodes are used to control the rhythm of cardiac activity, and for prosthetics for hearing problems.
But one cannot fail to mention the role of the “white metal” in other areas.
So, It is very much in demand in glass production.. Rather, not window glass, but high-quality optical glasses. Rhodium-platinum mix helps to make fiberglass dies with a thickness of less than 1 mm. For many thousands of hours, it functions properly at a temperature of 1400-1500 degrees, developing inside glass furnaces.
But platinum is also needed to create mechanisms that the glass industry uses. They are durable, do not oxidize with any reagents and do not react with the glass mass itself.
Elite Czech glass, which costs almost fabulous money, is made precisely inside platinum crucibles.

Of course, the chemical industry could not pass by such a substance resistant to heat and the most caustic reagents. In it crucibles are made from platinum, and other dishes for research and expert laboratories, for especially clean industries.
So, exactly based on Pt create some devices used to create semiconductor crystalsin. Only inside them can such conditions be created when the concentration of impurities is less than 1 atom per million. Even in platinum crucibles, they produce the crystals necessary for creating lasers and for contacts of low-current electrical engineering.

This metal goes on:
- mirrors used inside lasers;
- retorts for the production of hydrofluoric and perchloric acids;
- insoluble anodes for galvanic equipment;
- resistance thermometers;
- individual parts of microwave equipment;
- drugs that suppress certain forms of cancer;
- production of coins and insignia, medals and orders;
- equipment where vitamins and some other pharmacological preparations are synthesized.


How to choose a jewelry?
From the very beginning it is necessary to focus on the price. Platinum with an identical sample will be at least three times more expensive than gold. Moreover, there are not so many jewelers working with her. The vast majority of such masters work in Western Europe. It is advisable to focus on jewelry from there. But do not trust the price tag and the words of sellers, but require official certificates.
Additives of cobalt and ruthenium can increase the life of the product. Jewelry with the addition of iridium is often scratched, but can save money. The sample must be selected primarily taking into account its financial capabilities.
Important: all mechanized platinum jewelry is fragile and short-lived. Saving on the choice of products by masters working strictly by hand should not be.


And a few more tips:
- platinum is combined visually with any gems;
- the best option (if funds are available) would be to combine it with diamonds;
- inserted stones must conform to the general concept;
- engraved inscriptions and drawings can quickly go out of fashion;
- and, of course, you need to contact the official jewelry store, and not the first store you hit on the way or a kiosk in the middle of the underpass.

Care Features
There are no specific requirements here. Purification is usually carried out using special preparations for platinum. You can buy them at most jewelry stores. Some people use unsaturated soap or highly diluted ammonia. Sometimes liquid detergents are also used; however, soap and gels are thought to result in a loss of characteristic shine.
The most gentle option is washing with clean water and gently wiping with a soft cloth. Polishing at home is not possible.
It is carried out only by experienced jewelers on special equipment. The platinum product must be stored separately from jewelry made of other metals. This will protect them from deformation when touched by a harder object.


Interesting Facts
The high value of platinum is due not only to its own unique properties, but also to its comparative rarity. It is estimated that even in deposits (as well as in the entire earth's crust), the concentration of Pt is 30 times lower than the concentration of gold. From 1828 to 1845, platinum coins were made in our country. Their face value was 3, 6 and 12 rubles, and the total amount of metal consumed exceeded 14 tons. Platinum fell into the category of precious metals only in 1751 - approximately 200 years have passed since it became known in Europe.
But this metal is found not only on Earth. It was repeatedly found during chemical analysis of meteorites.
And in our country, in the very first 10 years of extraction, as much platinum was extracted as during the centuries preceding the discovery of the Ural deposits, they were mined throughout America. AND it is in Russia that both the largest, generally (re-melted), and the largest of the nuggets that have survived to our time are found. Platinum received the status of a chemical element in 1735, when the Italian D. Scaliger proved its indecomposability; previously believed that this is a simple substance.

Chemically pure platinum from ore was isolated only after 68 years in England. In metallic form, it is completely neutral biologically. However, individual compounds (primarily with fluorine) can be extremely life-threatening. And in 1867, all Russian platinum reserves (immediately after the lifting of the moratorium on sale) were bought by England. But in the early years of the twentieth century, our country accounted for at least 90% of world production (because fantastic reserves in southern Africa were found only in the mid-1920s).
It is not surprising, therefore, that in May 1918 a special institute for the study of platinum was created. Now it is part of the Institute of General and Inorganic Chemistry. Individual platinum-containing minerals also contain antimony, arsenic or sulfur, but they are less common than compounds with metals. Thus, the Norilskite mineral mined in the north of the Krasnoyarsk Territory includes 25% iron and 26% nickel. It is curious that at one of the stages of the industrial production of platinum, a sugar solution can be used.

And the names of this metal are quite diverse: they call it “rotten gold” and “frog gold”. Small crystals of it have a cubic shape. Initially, in the first half of the 19th century, rings and hoops for barrels were made from platinum in our country. In electrical conductivity, this metal is inferior to copper, and aluminum, and silver. Platinum begins to oxidize with atmospheric oxygen only at temperatures from 200 degrees.


Read more about platinum and its properties in the video.



